Life sciences
Transform your paediatric clinical trials
We help clinical research organisations convert, retain, and engage paediatric participants through better trial experiences and tailored preparatory support.
The problem
According to the FDA, 25-40% of paediatric clinical trials fail to provide data on the safety or efficacy of the study intervention. Lack of safety or efficacy data means the study intervention can’t be labelled as safe to use in paediatric settings. This often leads to high rates of off-label (79%) or unlicensed (35%) paediatric prescriptions.1
Recruitment, protocol adherence, and participant retention all affect the success of a paediatric clinical trial.

Paediatric clinical trial challenges
Poor recruitment
- $1200 is the average cost of a failed recruitment
Early withdrawal
- 20% of participants withdraw from clinical trials
Low adherence
- 50% adherence rate to prescribed medication
Delays
- 80% of clinical trials are delayed
Lost revenue
- $8 million lost in revenue each day a clinical trial is delayed
Our solution
Little Journey is a digital support platform that supports families through every step of their clinical trial journey, from initial screening to trial completion.
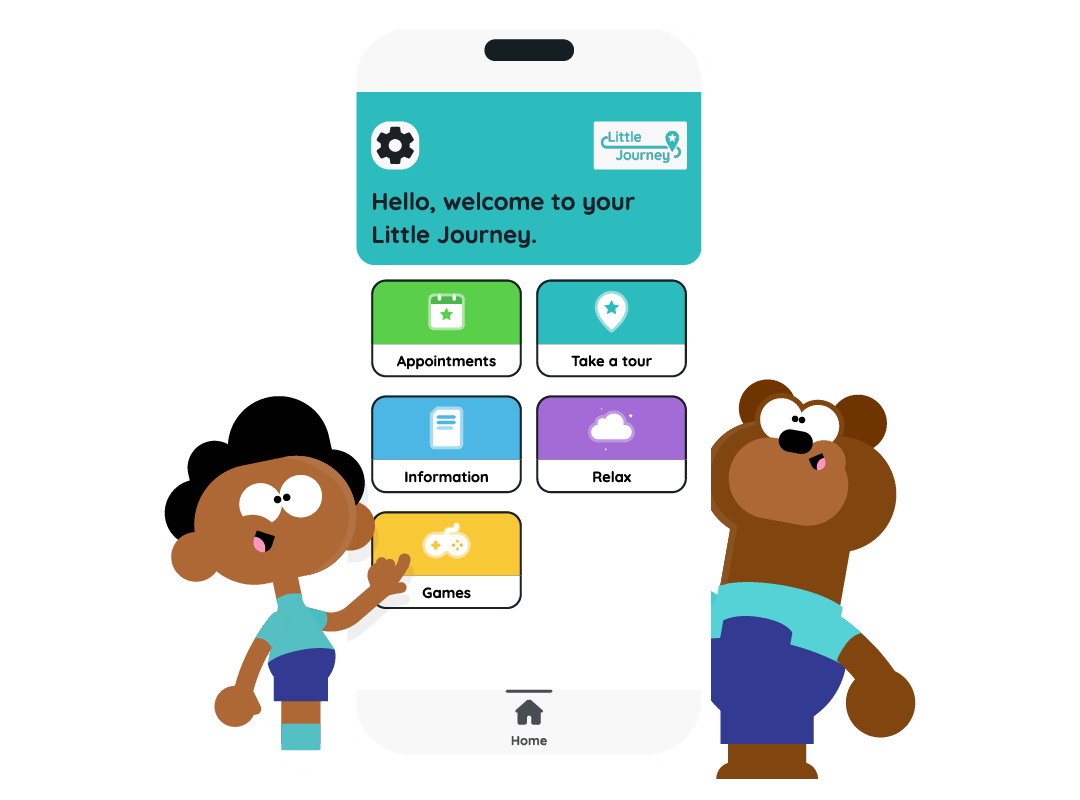
The Little Journey app offers:
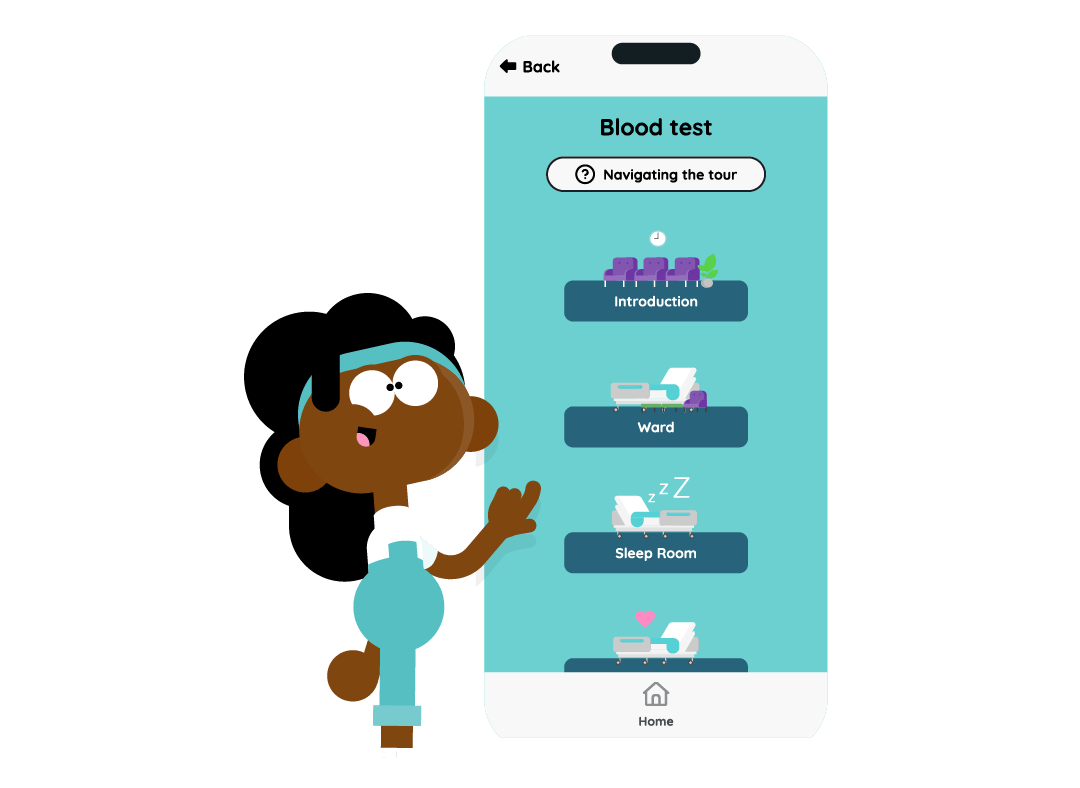
- Virtual site tours to explore the different rooms and learn about the clinical trial journey
- Bite-sized information articles to prepare and inform
- Distraction and soothing activities to cope with strong emotions
All configured to your organisation’s unique clinical trial!
Our web portal lets research staff monitor trial progress and configure our app content in real time to suit their organisation’s needs.
Tailored content
We provide interactive content tailored by age, procedure, and research site. We create content accessible to all children, with a strong focus on supporting neurodiverse children
Configurable by you
Our web portal lets research staff monitor trial progress and configure our app content in real time to suit their site’s needs. Adjust or amend our app content in only minutes!

Tailored procedure preparation content for:



Coming soon...
ECHO scans, ECGs, X-ray scans, Bone marrow biopsies or transplants, Intravenous infusions, Sleep studies
Why use Little Journey for your clinical trial?
Supporting participant recruitment
We provide each research site with the Little Journey platform, boosting pre- recruitment engagement with eligible participants.
Enhanced medication adherence
Our medication game playfully encourages participants in taking their medication. We support their home medication routines through nudge notifications and gamification techniques.
Improved participant experience
We provide families with psychological preparation and support content, so they feel better prepared for their trial. By doing so, we can improve participant satisfaction and experience of care.

Informed communication
We help support trial logistics and transform trial protocols into easily digestible information for your participants.

Higher participant retention
Positive experiences and trial engagement can support higher participant retention. We help research staff keep track of which families may need extra support during the trial.

Effective data capture
We provide research staff with a trial oversight through our in-app and behaviour data collection. This includes a breakdown of participant trial progress and site interactions.

Book a capabilities call
See how Little Journey can help your clinical trial
See more
References: (1) Pediatric Clinical Trials Market Analysis – Coherent Market Insights (2020). Online: Pediatric Clinical Trials Market Size, Trends, Shares, Insights, Forecast - Coherent Market Insights 2 - Rajeshwari Gore, Preeta K. Chugh*, Chakra D. Tripathi, Yangshen Lhamo and Sandhya Gautam, “Pediatric Off- Label and Unlicensed Drug Use and Its Implications”, Current Clinical Pharmacology (Discontinued) 2017; 12(1) . https://doi.org/10.2174/1574884712666170317161935


_screen%20use.png)

.png)
