Background
Little Journey partnered with a global pharmaceutical company to tailor our support solution for children with Duchenne Muscular Dystrophy (DMD) participating in gene therapy research.
DMD is a rare genetic disorder characterised by progressive muscle degeneration and weakness due to a fault in the dystrophin gene. Dystrophin is a protein that helps keep muscle cells intact.
Gene therapy is a new treatment which aims to introduce a working version of a gene into cells in place of the faulty one. For individuals with DMD, this therapy has the potential to protect the muscles and prevent disease progression.
Challenge
Screening & Enrolment
Gene therapy research presents unique challenges in screening and enrolment. Families pin their hope on the potential treatment, but not all will meet the inclusion criteria. For those who are eligible, enrolling means they forfeit any future gene therapy options. This creates a daunting "all or nothing" scenario for families. Our client needed to provide clear information and manage expectations sensitively, supporting ineligible participants while empowering eligible families to make informed enrolment decisions.
Engagement & Retention
Retaining participants during the monitoring phase is vital for successful data collection. However, this period brings uncertainty for families, who may worry about adverse events, effectiveness, and disruption to their child's life. If the burden feels too high for families, their dropout risk increases. Our client needed to offer ongoing support, motivation, and reassurance to keep families engaged throughout.
Our solutions
Family-centric information delivery
We developed support across five key stages of the gene therapy journey:
Support was tailored for children aged 2-7, children aged 8-12, and caregivers. Animated videos, articles, and interactive activities were chosen to deliver key medical concepts and research information in an age and developmentally-appropriate way.
73 topics were covered including ‘what is gene therapy?’, ‘what happens if my child isn’t eligible?’, ‘supporting emotions’, ‘celebrating bravery’, ‘medical site checklists’ and ‘why does my child need to be monitored?’.
.png?width=1437&height=1179&name=digital-physical-products%20(3).png)
Preparation and psychological support
Our established platform of digital content and offline resources was at the core. Developed with psychologists, child life specialists and medical writers, every resource uses evidence-based frameworks to help families prepare for, cope with and remain motivated throughout their clinical journey.
Co-creation
We collaborated with our ‘Little Leaders’ children’s advisory group, patient advocacy groups, and pharmaceutical industry experts to further understand the daily challenges families face with DMD and their questions around gene therapy. Through workshops, interviews, events, and surveys we gathered insights from 49 families to tailor our solution to rare disease gene therapy research.

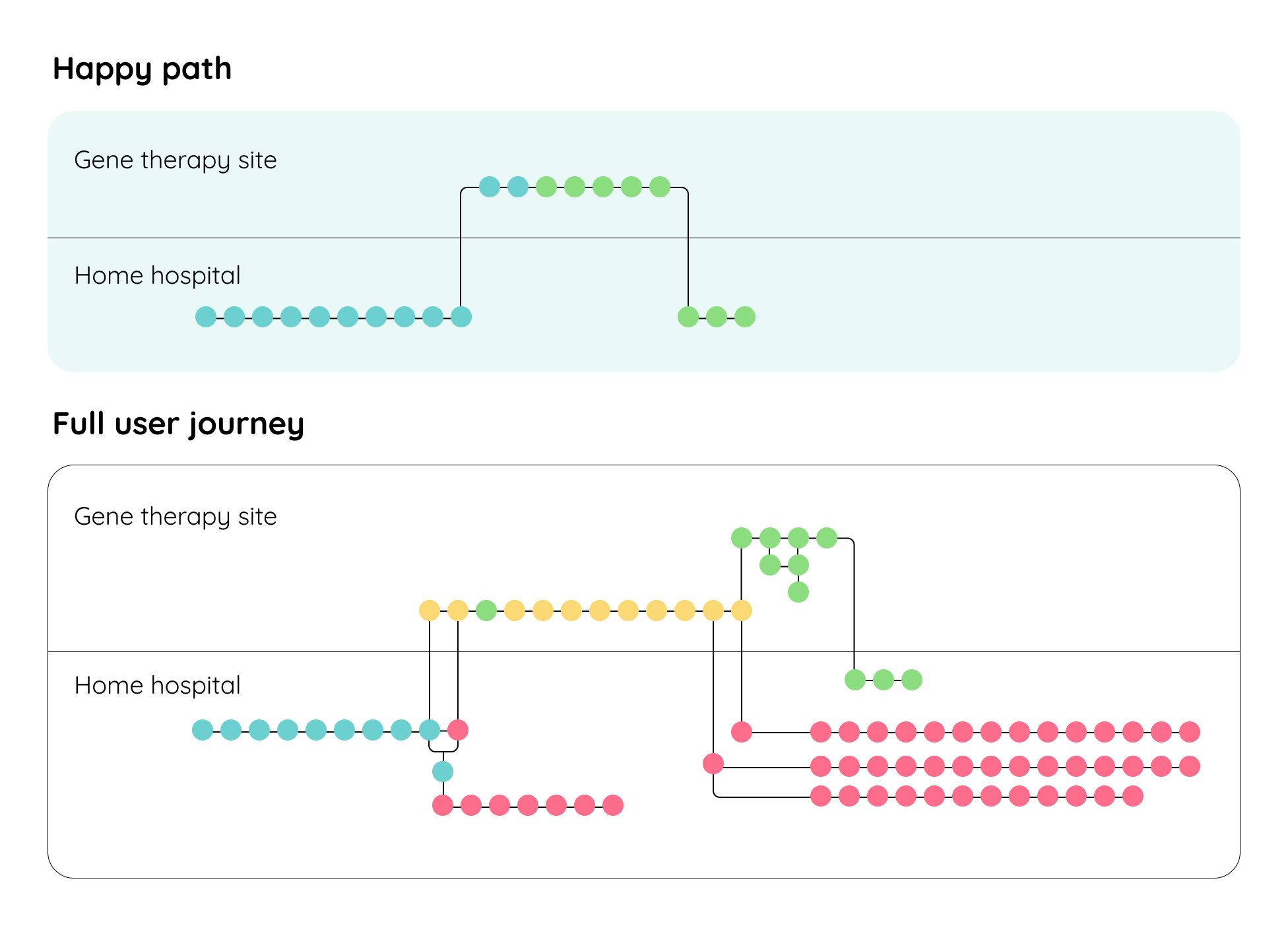
Happy vs. unhappy paths
These insights enabled us to map potential pathways that families may take on their gene therapy journey—including both ‘happy’ and ‘unhappy’ paths. We designed a wide range of resources so our solution could anticipate diverse need, supporting families at every step, whether it was expected or unexpected.
Outcomes
[Overview of the outcomes. Rearrange the cards below as needed!]
Little Journey's power wheel is this psychosocial aspect that is helping kids, and the parents as well. They are speaking to the gaps I cannot speak to.
Patient Lead
Pharmaceutical Client
Example early design mock-ups
We created over 50 mock-ups across different device types and screen sizes. This defined the best order, layout, and flow of support information across the five stages.
You may also like...
Case study
[Case study title]
[Summary of the case study, preferably a simplified version of the background or problem]

Case study
[Case study title]
[Summary of the case study, preferably a simplified version of the background or problem]

Case study
[Case study title]
[Summary of the case study, preferably a simplified version of the background or problem]



